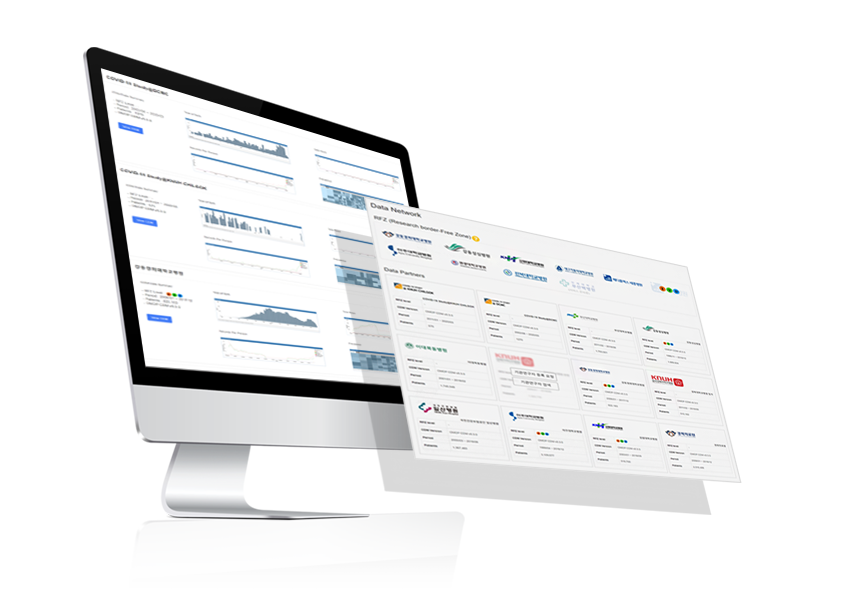
Multicenter collaborative research support
– Data network platform service
– Descriptive statistics and visualizations of partner hospitals’ data
- Remote study design and analyses

EVIX-ONE™ is a solution enabling multicenter
collaborative research leveraging EvidNet’s network.
Take a glance at descriptive statistics and visualizations
of partner hospitals’ data and remotely design and
execute studies. Conduct various analyses from simple
cohort definitions to complex comparative effectiveness
studies and AI modeling.

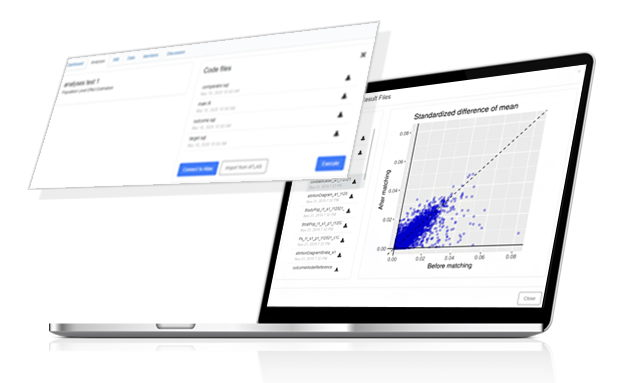
– Data network platform service
– Descriptive statistics and visualizations of partner hospitals’ data
- Remote study design and analyses
– Simultaneous execution of research codes at authorized sites
– View and download research analyses results
– Integrated R Shiny View

EVIX-MART™ is an on-demand ETL and data quality management service.
Based on our experience working with structured and unstructured
hospital EMR data, we can convert, expand, extend, and integrate data
from various sources including national claims data (HIRA/NHIS), national
statistics data (Statistics Korea), medical imaging data, genomic data and
patient generated health data (PGHD).
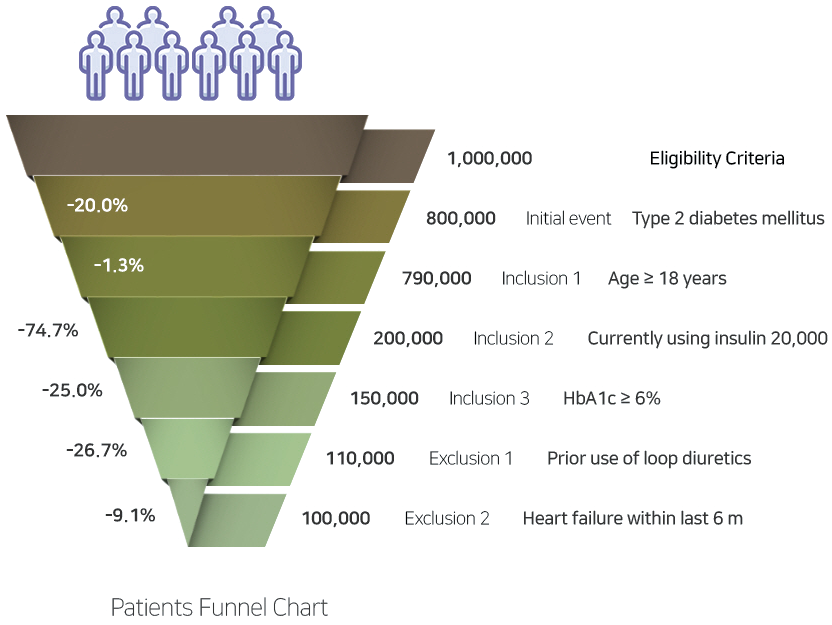

Through EVIX-FIND™, EvidNet provides fast-track
aggregated patient count query services for your
eligibility criteria of interest.
Leveraging our standardized data network of more than 30 hospitals in Korea, we can quickly identify the number of patients matching
your clinical trial-specific eligibility criteria.
Results are provided within 10 business days after receipt of request in the form of a report with relevant tables and funnel charts, enabling you to assess the impact of individual eligibility criteria. Breakdown of patient numbers per hospital and contact points at each hospital can also be provided should you wish to engage individual hospitals for patient enrollment into your clinical trial.
EVIX-FIND™ can streamline your clinical trial operations by reducing time and cost in finding eligible sites and patients and minimizing protocol amendments

EVIX-RESEARCH™ is an on-demand, contract retrospective
research service using EvidNet’s RWD/RWE Platform.
We can address all types of research questions, such as comparative effectiveness and safety studies,
postmarketing surveillance studies, change in specific lab test results after the administration of
a specific drug and predicting adverse drug reactions.
Through EVIX-RESEARCH™, you can review feasibility and anticipate the success rate of your clinical trials,
generate supportive evidence for traditional RCTs and prepare supplementary documentation for
submission to regulatory authorities such as MFDS.
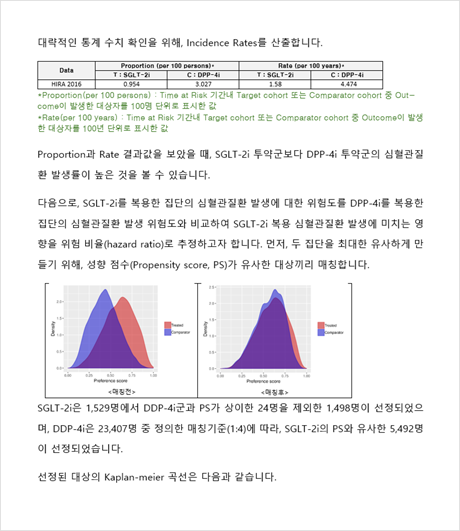
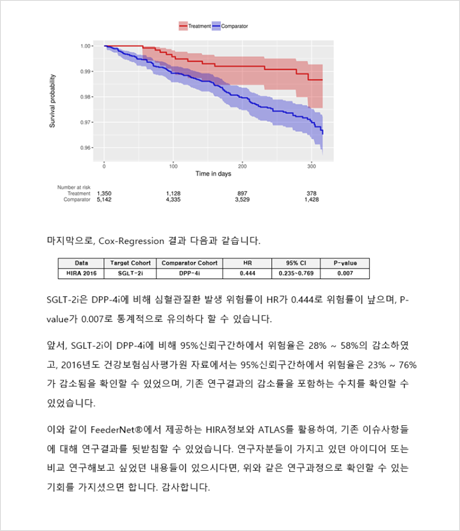

Depending on your external use and publication needs, EVIX-RESEARCH™ classified into two types: pilot study or full study. Study lead time varies depending on study type (pilot/full), study complexity and time to complete participating hospitals’ administrative procedures but a typical pilot study requires 1~3 months for completion.
Study results are provided in the form of a report with relevant tables and figures, including baseline characteristics table, patient flow diagram and statistical analysis results. In case of full study projects, we additionally support manuscript preparation for scientific publications.

EVIX-PV/PMS™ is an on-demand, postmarketing surveillance (PMS) service using EvidNet’s RWD/RWE Platform.
On December 16th, 2019, MFDS announced a revision of its 「Guideline on Re-Examination on Affairs of New Drugs, etc.」
In this revised guideline, “database studies” were officially added as a mean of postmarketing surveillance and is defined as studies using medical information databases, i.e. patient medical records, health insurance claims data, patient registration system and other data on patient health status collected over a period of time and systematically computerized. As per the new guideline, database studies may be conducted to detect or identify drug adverse events and information on drug effectiveness and safety.
Through EVIX-PV/PMS™, EvidNet provides RWD-based postmarketing surveillance services in compliance with these revisions.
* Please contact us for more information.

EVIX-EXPLO™ is a graphic user interface(GUI)-based exploratory data analysis (EDA) tool.
Using EVIX-EXPLO™, anyone can perform data analytic without the need for coding.
Conduct end to end analyses from dataset creation to viewing analysis results in a web environment.

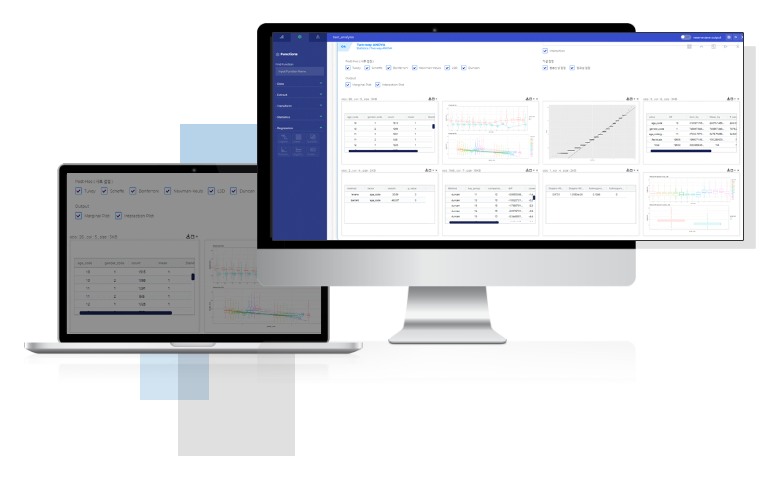
– Data preprocessing functions such as table joining, categorizing continuous data, filtering, etc.
– Statistics analysis functions such as basic statistics, normality tests, t-tests, proportion tests, ANOVA tests, correlation analyses, regression analyses, survival analyses, etc.
– Dynamic visualization functions to produce various charts and graphs
– Universal tool supporting not only data in CDM format but also data owned by individual researcher, pharmaceutical companies and CROs, as well as public data released by governmental organizations.

Experience RWE based on HIRA (national claims data) and hospital EMR data.
Gain various insights on top 500 diseases in Korea such as frequently
prescribed drugs, procedures, and lab tests

Do you require deeper RWE insights? Experience rich information
on specific cohorts of your interests. EVIX-INSIGHT PRO™ is planned for launch
in the second half of 2020.